Background: Acute myeloid leukemia (AML) is an aggressive hematological malignancy arising from an immature myeloid progenitor with notoriously poor outcomes. It has been established that the immune microenvironment has a significant impact on prognosis and has been targeted with therapy in other cancers. However, the immune microenvironment has yet to be fully characterized in AML, including changes as the disease progresses. This knowledge is critical for the development of immune based therapies for this disease since the patient's inflammatory and metabolic states may lead to failure of immunotherapy-based approaches. There is significant evidence that T-cell function is directly impacted by metabolism and inflammation. Therefore, we sought to evaluate and compare the metabolic as well as inflammatory states of patients with newly diagnosed and relapsed/refractory AML.
Methods: To assess metabolic and immune alterations associated with newly diagnosed (ND) (n=10) and relapsed/refractory (R/R) AML (n=10), we assessed and integrated metabolite and cytokine levels using de-identified, cryopreserved serum from Roswell Park. Research was conducted according to the Declaration of Helsinki in accordance with the Institutional Review Board regulated protocol. Metabolites were evaluated via the Biocrates MxP Quant 500 kit and assessed via both LC/MS-MS and Flow Injection analysis (FIA). For TCA metabolites, methanol was used to crash protein, followed by a derivatization with 3-Nitrophenylhydrazine (NPH), which was assessed on an Agilent 6545B LC/Q-TOF mass spectrometer. Descriptive statistics and graphics were used to evaluate metabolite concentration and immune factors by sample. Metabolite concentrations were then transformed if necessary to achieve a normal distribution. Only metabolites and immune factors that were reported in at least 10% of the samples were included in the analysis. A Limma-based, linear model approach was implemented for differential abundance. Associations between metabolites, immune factors, and clinical factors were assessed via correlation analysis, enrichment analysis, and network generation in Cytoscape.
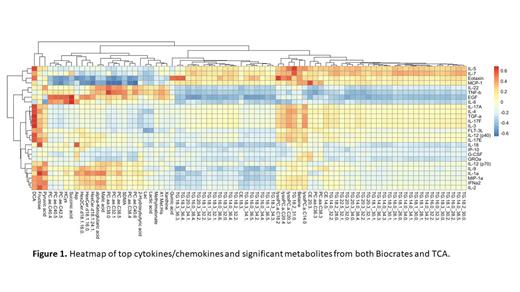
Results and Conclusion: The median age of the entire cohort (N=20) was 69 years (range: 38-92), with 11 female patients (55%). Two patient samples did not provide any signals on the cytokine panel so those samples were removed from the entire analysis (n=18 remaining samples). Analysis was adjusted for age and sex. 630 metabolites were measured utilizing Biocrates, however due to inadequate ability to quantitate several metabolites in all samples, a total of 581 metabolites were evaluated. We observed consistent alteration of several classes of lipids and alterations to amino acids/amino acid-related metabolites in ND compared to R/R. Statistically significant metabolite set enrichment was observed in methionine metabolism, malate-aspartate shuttle, purine metabolism, and phosphatidylcholine metabolism (P=0.03). Within the TCA metabolomics, 20 metabolites were quantifiable. Succinic acid and lactic acid were most highly upregulated in ND as compared to R/R patients. A total of 47 cytokines and chemokines were evaluated using the Luminex platform. We observed upregulation of MCP-1 and eotaxin in RR compared to ND. Dichloroacetate (DCA) which has been associated with immune regulation of T cells, was found to be significantly associated with a large number of immune factors (Figure 1). Furthermore, significant correlation was observed between IL-6 and overall survival (OS) as well as succinic acid and OS. To confirm these findings, an additional 10 ND and 10 R/R patient samples are currently undergoing evaluation utilizing these methods in addition to T cell functional studies.We demonstrate significant alterations within metabolism and inflammation in ND vs. R/R AML patients, which suggests that the immune microenvironment undergoes significant change upon disease progression/relapse. ND patients appear to have more robust metabolic profile, suggestive of a healthier, more functional immune milieu as compared to R/R patients which appear to be demonstrating a more immune-exhausted phenotype.
Disclosures
Przespolewski:Jazz Pharmaceuticals: Research Funding. Davila:Adaptive Biotechnologies: Other: Ownership interest (stock, stock options in a publicly owned company); Adicet: Consultancy; Atara Biotherapeutics: Consultancy; Bellicum Pharmaceuticals, Inc.: Other: Advisor or review panel participant; Ownership interest (stock, stock options in a publicly owned company); CRISPR (CRSP): Patents & Royalties: Intellectual property rights (Royalties or patent sales); Capstan: Other: Advisor or review panel participant; Caribou Biosciences: Consultancy; Kite Pharma Inc.: Other: Teaching and Speaking; Legend Biotech: Consultancy; Precision Biosciences: Other: Ownership interest (stock, stock options in a publicly owned company); Syncopation Life Sciences: Consultancy; Synthekine: Consultancy.